Abstract
Introduction
Bleeding is the major complication of anticoagulant therapy. Reversal of vitamin K antagonists (VKAs) in patients with major bleeding events (MBE) including intracranial haemorrhage (ICH) is achieved with prothrombin complex concentrate (PCC) and intravenous vitamin K. Administration of PCC or activated PCC is approved UK strategy for treating MBE associated with rivaroxaban or apixaban, pending approval of a specific reversal agent. In all cases there is concern regarding the incidence of thrombotic events.
Methods
In this respective review, the efficacy and safety of 4-factor PCCs for the management of MBE in 344 patients receiving rivaroxaban, apixaban or warfarin were compared from January 2016 to April 2018.
All patients had full blood count, coagulation screen (INR for VKA) and biochemistry profile at presentation and prior to receiving PCC. Patients on rivaroxaban or apixaban had samples taken for drug level but did not wait for the results to administer PCC. Concomitant antiplatelet or nonsteroidal anti-inflammatory drugs, length of stay, thromboembolic events, re-bleeding at the same site and 30-day mortality were documented from medical records.
Results
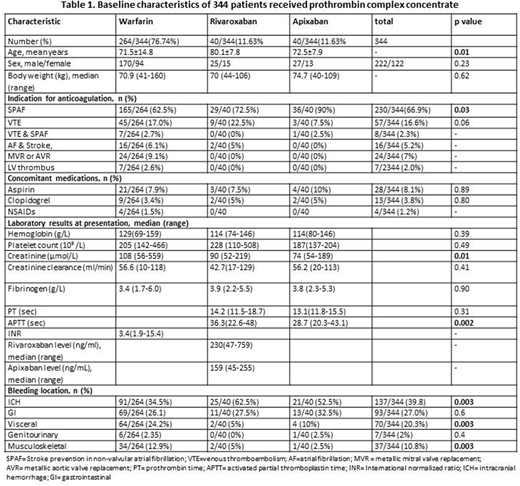
Table 1: Baseline characteristics of the 344 patients
Overall median (range) dose of PCC was 2000 units of Factor IX (500-5000). As expected, AF was more frequently the indication for apixaban and rivaroxaban (36/40, 90% and 29/40, 72.5%) than it was for warfarin (165/264, 62.5%). Patients with metallic heart valves or left ventricular thrombus received only warfarin. ICH was the most common indication for PCC in all three anticoagulant groups (137/344, 39.8%) followed by gastrointestinal bleeding (93/344, 27%). ICH was more frequently the indication for PCC in patients receiving rivaroxaban (25/40, 62.5%) and apixaban (21/40, 52.5%) compared to warfarin (91/264, 34.5%), p=0.003. Patients on warfarin more frequently received PCC for musculoskeletal bleeding (12.9%) compared to those on rivaroxaban (5%) and apixaban (5%), p=0.003. There were no differences in the other bleeding sites based on type of anticoagulant.
No differences were observed in hemoglobin, platelet count and fibrinogen in patients based on type of anticoagulant or site of bleeding. Median (range) rivaroxaban and apixaban levels at time of receiving PCC were 230ng/ml (47-759) and 159ng/ml (45-255) respectively. Median INR pre- and post-PCC in patients on warfarin were 3.4 (1.9-15.4) and 1.2 (1.0-1.9) respectively. A significantly higher proportion of patients on apixaban (17/40, 42.5%) or rivaroxaban (14/40, 35%) received tranexamic acid compared to warfarin (51/264, 19.2%), p=0.001. Vitamin K was given to 227/264, 86% of patients on warfarin with median dose 5mg (range 1mg-10mg). There was no difference in number of red cell units, platelet or FFP used in the three anticoagulant groups. Overall 30-day mortality rate was 24.7% (85/344) and did not differ according to type of anticoagulant (p=0.17). Recurrent bleeding occurred in 18% overall, warfarin 15.8%, rivaroxaban 20% and apixaban 30.8%, p=0. 07. Recurrent thrombosis occurred in 4.1% (14/344) patients within 30 days of receiving PCC with no difference in three anticoagulant groups, p=0.83 (warfarin 4.2%, rivaroxaban 5.1% and apixaban 2.5%). Nearly half of the patients (48.1%), restarted anticoagulation in a median 13.5 days (6-44). Anticoagulation was restarted in a significantly higher proportion of patients on warfarin (138/264, 52.3%) compared to (10/40, 25%) on rivaroxaban and (13/40, 32.5%) on apixaban, p=0.001.
In patients with ICH there were no differences in age or sex between groups. There were no differences in concomitant use of antiplatelet treatment, recurrent bleeding (overall 17.8%, warfarin 17.8%, rivaroxaban 20% and apixaban 15%, p=0.90) or 30-day mortality (overall 30-day mortality was 35%; warfarin 31.9%, rivaroxaban 44% and apixaban 38.1%, p=0.50). None of the patients with ICH on rivaroxaban or apixaban had thrombotic events and only 2/91 (2.2%) patients on warfarin had ischemic strokes within 30 days post PCC. Overall 30.6% of patients with ICH restarted anticoagulation with a median 15 days (10-42).
In conclusion, there was no difference in the safety (thrombosis) and efficacy (30-day mortality and re-bleeding) in use of PCC to treat MBE in patients on warfarin, rivaroxaban and apixaban. Some differences may reflect indications for anticoagulation.
Jayakody Arachchillage:Octapharma: Other: Sponsored to attend an educational meeting; Mitsubishi Phama: Other: Sponsored to attend an educational meeting; Bayer: Other: Sponsored to attend educational meetings. Laffan:Roche: Consultancy, Speakers Bureau; Pfizer: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal